Abstract
Background: Non-vitamin-K-antagonist oral anticoagulants (NOACs) have rapidly become a cornerstone in anticoagulant therapy. However, anticoagulated patients older than 80 years represent an especially challenging population, since their thromboembolic and bleeding risks are excessively high and chronic kidney disease is common, making treatment decisions even more complicated. As a consequence, long-term safety data for this specific population are needed.
Patients and methods: Using data from DRESDEN NOAC, a prospective regional registry in Germany in which patients with oral anticoagulation undergo prospective follow-up (FU) by quarterly phone interviews, we assessed rates of thromboembolic and bleeding outcomes in patients older than 80 years, based on centrally adjudicated events using standard outcome definitions.
Results: Until 30th November 2017, 3984 patients were enrolled into the registry (53% male, mean age 70.2 years), including 935 patients aged ≥ 80 years (487 [52.1%] received rivaroxaban, 198 [21.2%] apixaban, 165 [17.6%] edoxaban and 85 [9.1%] dabigatran, respectively). Indication for anticoagulant therapy was atrial fibrillation (AF) in 752 (80.4%) cases, venous thromboembolism (VTE) in 179 (19.1%), and other indications in 4 (0.4%) cases. This subset of patients had a mean age of 84.2 years (range 80-100y) and 406 (43.4%) patients were male (Table 1).
During a mean follow-up of 970.7 ± 642.2 days (mean NOAC exposure 815.3 ± 644.5 days) an intention-to-treat (all events counted) analysis of all very old patients demonstrated an event rate for the composite endpoint of stroke, TIA, systemic embolism or VTE of 1.0/100 patient-years (95% CI 0.8 - 1.2). In AF patients, the rate for stroke, TIA, systemic embolism was 1.0/100 patient-years (95% CI 0.8 - 1.2) and in VTE patients, the rate for recurrent DVT or PE in VTE patients was 0.4/100 patient-years (95% CI 0.2 - 0.7).
In the on-treatment analysis (only on-treatment events counted), the corresponding event rates were 0.7/100 patient-years (95% CI 0.5 - 0.9) for the composite endpoint, 0.7/100 patient-years (95% CI 0.5 - 0.9) for stroke, TIA, systemic embolism in AF patients, and 0.1/100 patient-years (95% CI 0.01 - 0.3) for recurrent VTE in VTE patients, respectively. The overall rate of ISTH major bleeding was 1.0/100 patient-years (95% CI 0.8 - 1.2), with numerically higher rates in SPAF patients (1.2/100 patient-years; 95% CI 0.9 - 1.5) compared to VTE patients (0.4/100 patient-years; 95% CI 0.2 - 0.7).
255 patients died during FU (2.2/100 patient-years; 95% CI 2.0 - 2.5), of which 120 deaths occurred during or within 3 days after last intake of NOAC (1.3/100 patient-years; 95% CI 1.1 - 1.6). Most common causes of death were fatal cardiovascular event (n= 86; consisting of 20 cases of acute coronary syndrome, 19 ischaemic strokes, 17 VTE, 2 systemic embolism and 123 cases of other cardiovascular deaths such as worsening of chronic heart failure, or unexplained deaths ruled as potentially related to cardiovascular events) and age related death (n= 79), followed by sepsis/infection (n= 40), terminal malignant disease (n= 26), fatal bleeding (n= 11) and other causes (n= 13).
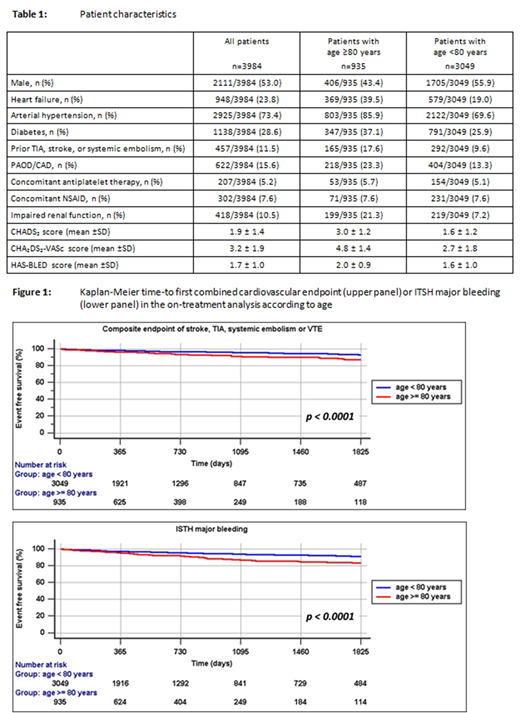
Overall, after 1800 days of FU, approximately 80% of this very old population were outcome-free survivors, as indicated by the Kaplan Meier curve (figure1).
Conclusions: During long-term FU of more than 2.5 years, this very old population of NOAC recipients demonstrated low rates of cardiovascular or major bleeding complications during active NOAC therapy. Approximately one quarter of the study population died during follow-up, with cardiovascular events being the leading cause of death. Only 11 fatal bleeding events were observed; however, most of the 58 fatal thromboembolic events occurred after anticoagulation was discontinued. This indicates that continued anticoagulation with NOACs may result in a beneficial risk-benefit ratio also in very old patients.
Beyer-Westendorf:Bayer: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding. Marten:Bayer: Honoraria; Daiichi Sankyo: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal